Oral Presentation The Annual Scientific Meeting of the Endocrine Society of Australia and the Society for Reproductive Biology 2013
Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes mellitus: a randomised controlled trial. (#118)
Background:
Up to 50% of men with type 2 diabetes (T2D) have lowered testosterone levels. Cause and effect are debated and whether testosterone therapy improves glucose metabolism is not well established (1).
Methods:
We conducted a randomised, double blind, placebo-controlled, 40-week trial of intramuscular testosterone undecanoate in men with T2D, with glycated haemoglobin (HbAlc) ≤8.5% and a serum total testosterone (TT) of ≤ 12nmol/L (ClinicalTrials.gov, number NCT00613782). No changes to oral hypoglycemic therapies were permitted. The primary outcome measure was a change in insulin resistance (HOMA-IR).
Results:
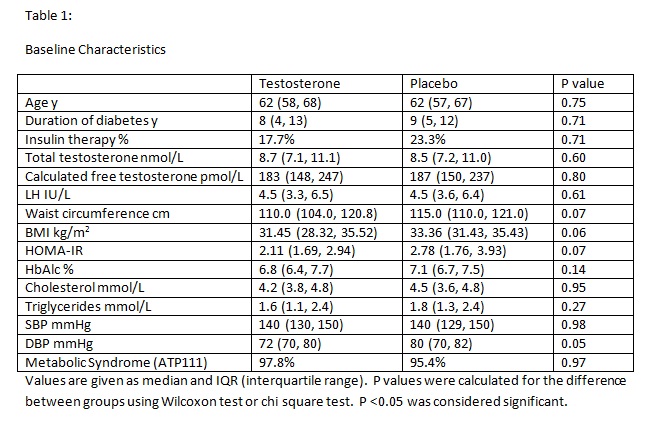
88 men were randomised and 75 completed the study. Baseline characteristics are shown in table 1.
In the testosterone (T) group at 40 weeks, TT increased (+4.6nmol/L p<0.001 IQR [0.7,8.8]) while there was no significant change in the placebo group. Testosterone therapy had no significant effect on HOMA-IR (40 week adjusted between group difference -0.08 p=0.23 CI [-0.31, 0.47]) or HbA1c (+0.36 p=0.05 CI [0, 0.7]). There were no significant changes in either group in fasting glucose, dynamic insulin resistance as measured by oral glucose tolerance test, anthropometry, lipid profile, blood pressure or change in the prevalence of metabolic syndrome. In the T group, changes in circulating TT during treatment correlated inversely with change in total body fat mass (r=-0.36, p=0.02) while there was no correlation with HOMA-IR (r=0.13, p=0.47). In men treated with testosterone, haematocrit (HCT) increased by +0.04 % (p < 0.001 IQR [0.02, 0.07]) and to 0.54 % or above in 5 subjects (11%).
Conclusions:
In men with T2D and lowered serum testosterone, testosterone therapy was not associated with significant improvements in measures of glucose metabolism or the metabolic syndrome despite altering body composition in a metabolically favourable manner.
Disclosure: This is an investigator initiated research trial with funding and trial medications provided by Bayer Healthcare. Bayer Healthcare was not involved in design, conduct or analysis of this trial.